Page 16 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 16
Original Scientific Article
122
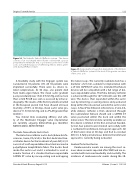
Figure 16: The Embrella Embolic Deflector system. The device consists of an oval-shaped nitinol frame covered with a porous polyurethane membrane that is positioned at the level of the aortic arch with the purpose of deflecting embolic debris gener- ated during TAVR procedures.
A feasibility study with the Engager system was conducted in 10 patients [39]. All 10 patients were implanted successfully. There were no device re- lated complications. At 30 days, one patient died from multi-organ failure. The mean aortic gradient post-procedurally was 15.6± 4.9 mm Hg, and no more than a mild PV-AR was seen as assessed by echocar- diography. The results of the first 61 patients enrolled in the European pivotal trial have showed all-cause mortality of 9.9% at 30 days, mean aortic valve gra- dient of 11.5±5.0 mm Hg, and no PV-AR greater than mild [41].
Two clinical trials evaluating efficacy and safe- ty of the Medtronic Engager valve implantation are currently ongoing (ClinicalTrials.gov Identifier: NCT01348438, NCT01789567)
The Helio Transcatheter Aortic Dock
The Helio transcatheter aortic dock (Edwards Life- sciences, Irvine, CA, USA) is the first dedicated tran- scatheter device for the treatment of pure AR [42]. It consists of a self-expandable nitinol stent encased in polyethylene terephthalate fabric. The dock is fixed inside the aortic root and it is intended to assist in annular fixation of a standard balloon-expandable SAPIEN XT valve by incorporating and entrapping
Figure 17: Angiographic image after deployment of the Embrel- la Embolic Deflector system at the level of the greater curvature of the aortic arch.
the native cusps. The currently available dock has a diameter of 25 mm, suitable for implantation with a 29 mm SAPIEN XT valve. It is intended that future devices will be compatible with a full range of bal- loon-expandable valves. The Helio delivery catheter is advanced through the 16 Fr eSheath over the stiff wire. The dock is then expanded within the aortic root by retracting a covering sleeve and positioned deep within the sinuses but outside the aortic valve cusps. A NovaFlex (Edwards Lifesciences, Irvine, CA, USA) delivery catheter is then advanced through the contralateral femoral sheath and a SAPIEN XT valve positioned within the dock and within the nativevalve.Theclinicaldatacurrentlyavailableon this device is limited. In the first-in-human feasibili- ty trial, four patients were treated successfully with a combined transfemoral-transapical approach. All of them were alive at 30 days and had no residual AR [43]. A fully percutaneous bilateral transfemoral approach is currently being evaluated.
Cerebral Protection Devices
Cerebrovascular events are among the most se- rious adverse events reported after TAVR and are as- sociated with increased morbidity and mortality. The incidence of cerebrovascular events during the 30-
Structural Heart Disease, August 2015
Volume 1, Issue 3: 112-126